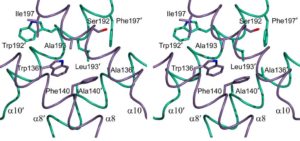
Design of a chain-specific heterodimeric variant of TetR
The specificity and selectivity of protein-protein interactions are of central importance for many biological processes. We used the in-house side-chain packing program MUMBO to computationally design a chain-specific heterodimeric variant of the bacterial transcription regulator tetracycline repressor (TetR), called T-AB. Our goal was to engineer two different TetR chain variants, A and B, that no longer interact as AA or BB homodimers but selectively recombine to form heterodimers. Although 56 residues from each chain contribute to a dimer interface as large as 2200 Å(2) in wild-type TetR, the substitution of only three residues in one chain and two residues in a second chain sufficed for generating specificity in a T-AB heterodimer variant. The design was corroborated in vivo by a cell-based transcription assay, and in vitro by CD spectroscopy and X-ray crystallography.
J Mol Biol. 2010; 403: 371-385 (PMID: 20816982).