pUL50-pUL53
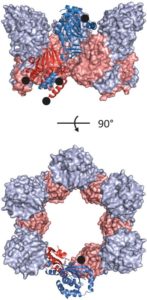
Nuclear replication of cytomegalovirus relies on elaborate mechanisms of nucleocytoplasmic egress of viral particles. Hereby, the role of two essential and conserved viral nuclear egress proteins pUL50 and pUL53 is pivotal for the formation of the core nuclear egress complex (NEC). Here, we report the crystal structure of the pUL50-pUL53 heterodimer (amino acids 1-175 and 50-292, respectively) at 2.44 Å resolution. pUL53-specific features include a zinc-binding site and a hook-like N-terminal extension, the latter representing a hallmark element of the pUL50-pUL53 interaction. A close examination of the crystal structure indicates assembly of pUL50-pUL53 heterodimers to hexameric ring-like structures possibly providing additional scaffolding opportunities for NEC. Combined, the structural information on pUL50-pUL53 considerably improves our understanding of the mechanism of HCMV nuclear egress and may accelerate the validation of the NEC as a unique target for developing a novel type of antiviral drugs.
J Biol Chem. 2015; 290: 27452-27458 (PMID: 26432641).